C-terminal Modifications
A wide variety of C‑terminal modifications can be prepared by JPT's peptide synthesis group. Typical examples are shown in the following table. Other examples include the C-terminal attachment of thiols (Cys side chain), biotin (Lys or Cys side chain), Abz (Lys side chain) and other labels or dyes.
The default C-terminus of a peptide is either a free carboxylic acid or an amide. When a peptide is meant to imitate part of a parental protein sequence, the more “native” end relates to a C-terminal amide. In addition, this modification avoids the introduction of additional charges in the peptide molecule.
|
Modification |
Structure | Applications/Comments |
|---|---|---|
|
Acid |
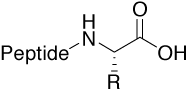 |
Standard (charged C-terminus) |
|
Amide |
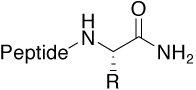 |
Standard (uncharged C-terminus) |
|
Ester |
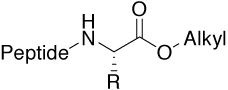 |
For structure-activity relationships (SAR), |
|
Aldehyde |
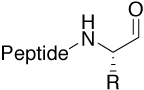 |
Reactive intermediate, e.g. for non-native chemical |
|
pNA (para-Nitroanilide) |
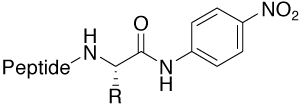 |
Protease substrate furnishes UV active pNA (405 nm) |
|
Amc (7-amino-4-methylcoumarinyl) |
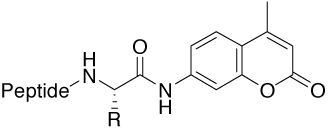 |
Tools for studying proteases (activity and specificity) |
|
Hydrazide |
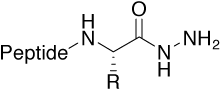 |
Metal-binding structural motif found especially in |
|
Hydroxamic acid |
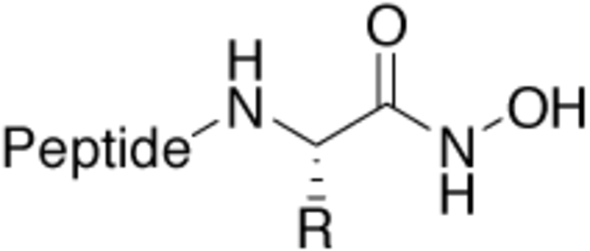 |
Zinc and iron binding structural motif, especially in |
|
Chloromethyl ketone (CMK) |
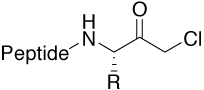 |
Motif in irreversible protease inhibitors. Availability |
