Peptide Quality & Purity
1. Peptide Purity
Depending on the target specification, synthetic peptides may contain varying amounts of impurities. These include truncated or deletion peptides, protecting group artifacts, partially oxidized, hydrolyzed, rearranged or decomposed products, residual chemicals and other impurities. Even small impurity levels may create significant problems in your assay. However, the impact of such by-products strongly depends on your specific application where it is essential to choose the appropriate peptide purity tailored to your application. Tell us about your application, and we will help you select the best specification for your peptides.
In addition and independent from the peptide purity, JPT applies critical quality measures in its manufacturing processes to avoid the mentioned impurities. These measures, applied to all peptides, minimize the formation of false positive T cell responses caused by de novo formed epitope peptides as well as inhibition of cellular responses by residual toxic chemicals.
Peptide purity is routinely determined by HPLC (high performance liquid chromatography). This method enables separation and relative quantification of each peptide synthesized. If coupled to a mass spectrometer (MS), this provides molecular mass/ identity information of the target peptide and accompanying impurities.
Therefore, HPLC-MS is the standard analytical tool at JPT applied for each peptide (except Fast Grade peptides).
In addition, JPT provides a wide range of additional analytical methods for high-resolution proof of the peptide composition, and quantified content of residual chemicals and biologicals.
Have a look at our Peptide Analysis Services for more information.
JPT's Peptide Purity Grades for immunology products
Fast Grade | Fast Plus Grade | Research Grade | Research Plus Grade | Development Grade | Trial Grade | Clinical Grade* | |
| Purity | unpurified (crude) | unpurified (crude) | each peptide is detectable | each peptide is guaranteed to be the main product | each peptide is purified to > 70% | each peptide is purified to > 80% >90% or >95% | each peptide is purified to >90% or >95% |
| Example applications | T cell epitope discovery, target discovery | neo-epitope prioritization, target identification, screening | T cell epitope discovery, immunogenicity testing, identification of immunodominant antigens | T cell epitope discovery, target identification, screening | immune monitoring, T cell epitope mapping | immune monitoring in trials, vaccine development | clinical immune monitoring, diagnostics and therapy development |
| QC | statistical HPLC-MS (5% of all peptides) | HPLC/MS of each peptide | HPLC/MS of each peptide | HPLC/MS of each peptide | HPLC/MS of each peptide | HPLC/MS of each peptide | HPLC/MS of each peptide + further QC and documentation |
| Synthesis method | high-throughput SPOT synthesis | high-throughput
SPOT synthesis | resin-based peptide synthesis |
resin-based peptide synthesis |
resin-based peptide synthesis |
resin-based peptide synthesis |
resin-based peptide synthesis |
| Related products | PepTrack Fast Track SpotMix | PepTrack Fast Track Plus SpotMix Plus | PepTrack Research Track | PepTrack Research Track Plus PepMix Research Plus Grade | PepTrack Development Track PepMix Development Grade | PepTrack Trial Track PepMix Trial Grade Antigen Peptides | Clinical Peptides & Pools |
* Clinical Grade Peptides are synthesized in an enhanced peptide synthesis environment including a variety of quality features required for clinical peptide applications.
2. Peptide Quality Measures Beyond Purity
ISO 9001 certification

Since 2014, we maintain a certified quality management system (QMS) that is audited annually by an independent certification body. Our QMS regulates standard operating procedures for reproducible workflows, deviation management, batch documentation, CoA's, data storage and more.
For further information have a look at our quality management system page.
We invite our valued clients to visit JPT and audit our quality system to assure highest quality for their out-contracting needs.
Animal-derived component free (ADCF)
We work according to an animal-derived component free (ADCF) policy for all of our peptides.
Animal sourced raw materials are primarily a concern regarding Transmissible Spongiform Encephalopathy (TSE) and viral contamination. We minimize these risks by selectively sourcing raw materials and producing products classified as Animal Component Free (ACF).
Our measures include:
- Equipment which has not been exposed to animal-derived materials or has been decontaminated
- Use of non-animal derived raw materials
- Manufacturing takes place in laboratories where no materials of animal origin are stored and used
- Other components, tools and processes used to decrease the risk for contaminating peptides
Non-peptide impurities
We purify all peptides with a guaranteed purity level irrespective of whether the crude material already meets the target purity or not. This leads to a significant reduction of many unwanted contaminants even beyond peptide impurities such as residual solvents, chemicals from the synthesis process and excess of trifluoroacetic acid (TFA) that might influence your assay.
TFA Content & Counter Ion Exchange
After synthesis and purification, peptides are present in form of their trifluoroacetate (TFA) salts.
TFA is used during peptide cleavage and purification by HPLC. Whereas free TFA is removed during workup and freeze-drying, it also protonates side chain amino functionalities of amino acids and the free N-terminus of the peptide. TFA anions represent the counter ion for each of the resulting positively charged amino functionalities. Those anions are present in sub toxic concentrations and usually buffered by your reconstitution medium.
However, if your assay/application is sensitive against TFA salts, we are able to remove the TFA salts completely by exchanging the TFA anion with chloride or acetate.
As efficiency of the TFA anion exchange is dependent on the amino acid sequence of each individual peptide, we plan counter ion exchange carefully, whereby we consider several topics:
| Risk | Counter measure |
|
Decomposition of peptides
|
Post counter ion exchange peptide analysis is mandatory for each peptide (i.e. by HPLC-MS) as partial decomposition may occur
|
|
Counter ion exchange is sequence dependent
|
Apply optimized and peptide sequence-specific protocols
|
|
Incomplete ion exchange
|
Determine residual TFA quantitatively after exchange for each peptide
|
Difficult Peptides
State of the art peptide synthesis and purification protocols enable efficient assembly of most peptides up to a chain length of 50 amino acids.
However, some peptides (up to 10%) give rise on difficulties to make them accessible in sufficient yields and purities. Such difficulties are peptide sequence-dependent and may result from:
- Strong hydrophobicity
- Secondary structure propensity
- Spontaneous re-arrangement and oxidation reactions
- Hydrolysis and peptide bond cleavage
- Loss of stereomeric integrity
- Remaining protecting group artifacts
- Several more
Contact us to learn more about how we are able to synthesize difficult peptides!
Average Purity
Product descriptions from other vendors might list an “average” or “mean” purity for peptide pools or peptide libraries. This can be misleading since some individual peptides in the pool might have a very low purity (or are even missing), while this is compensated by peptides having a high purity creating the average.
At JPT, for peptides offered with a specific purity (e.g. >70% or > 90%), we always provide the guaranteed minimum purity for each individual peptide in the pool or library.
You can trust that each peptide is at or above the target purity specification!
Peptide Pool Analysis
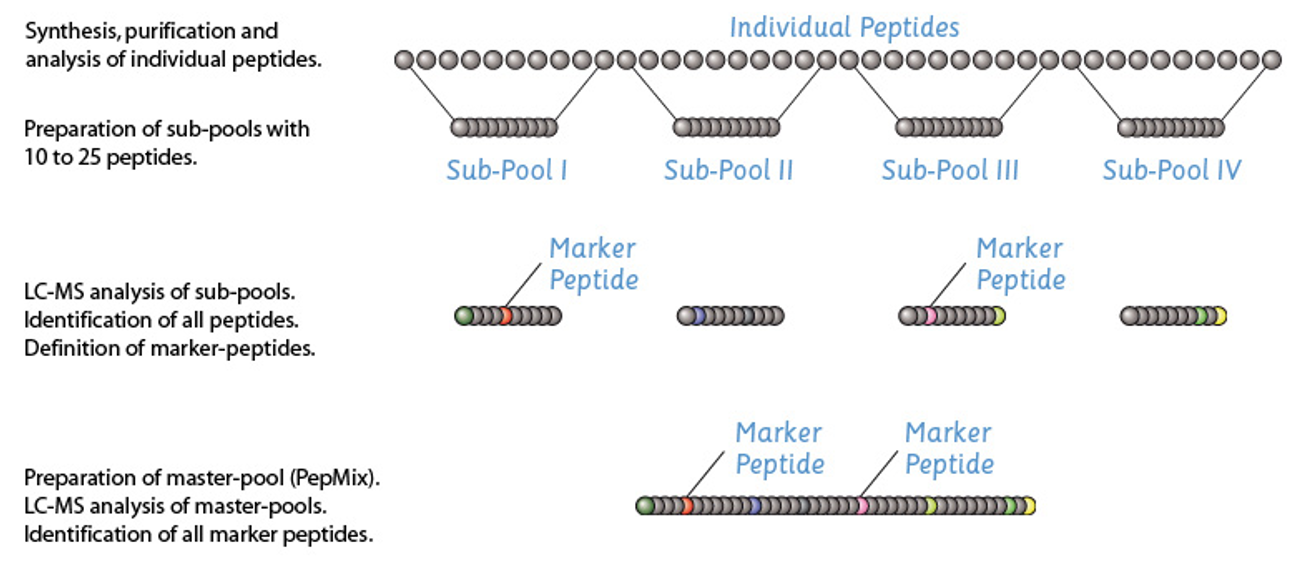
Our peptide pools can be complex and contain up to several hundred of individually manufactured peptides.Presence of each peptide in the pool is critical to warrant proper function and for quality and regulatory compliance. But even state-of-the-art analytical methods such as HPLC-MS or UPLC-MS are not always able to deconvolute all peptides in complex peptides mixtures. Therefore, we developed a proprietary analysis workflow that provides proof of the presence of all peptides within a peptide pool. You will receive documentation of this analysis with your customized peptide pools and can request it for our catalog peptide pools displaying a purity of 70% or greater.
Manufacturability Algorithm
We have developed a proprietary algorithm called PepSelector to evaluate manufacturability and feasibility of peptide sequences based on experimental data from several tens of thousands of synthetic peptides and known motifs prone to influence peptide manufacturability and stability.
The goal of applying this algorithm is to predict potential difficulties and rank a given set of peptides according to their overall manufacturability. Therefore, in time sensitive studies such as neo-epitope based individualized immunotherapies, we can ensure efficient and successful synthesis. In addition, the program provides indication of potential solubility and stability problems and is able to suggest optimization of sequences by shortening, extension or frame shifting the peptide sequences along their parental antigen sequences.
For further information, please visit the Manufacturability Evaluation/Ranking.
Line clearance
Cross-contamination of synthetic peptides and peptide pools with unrelated peptides is not uncommon and represents a serious risk for assay performance and regulatory compliance.
The reason is that peptide vendors process many orders at the same time in parallel. Depending on the quality system and SOP’s in use, these orders are executed in the same working space using the same peptide synthesizer, the same HPLC column for purification, the same freeze-drying instrument etc. Thus, you need to apply mitigating measures to avoid cross-contamination.
At JPT, we have different quality levels that address the mentioned issue at three levels. Depending on the quality level (RUO, ISO PLUS or CGP) our measures include application of thoroughly cleaned or new laboratory items, of dedicated instrumentation, specialized cleaning procedures, organizationally or spatially separated laboratory environments and working spaces and more.
Low Bioburden Manufacturing Processes
Manufacturing of peptides usually does not take place in a sterile environment. Therefore, peptide products per se are non-sterile.
However, since JPT is mainly using organic solvents for its manufacturing processes, the workflows are low bioburden processes, which we have shown by numerous sterility and bioburden tests that prove sterility of JPTs products.
In addition, we apply various measures to minimize the risk for biological contaminations:
- Established pest control and hygienic program
- Cleaning procedures prior to specific process steps during the manufacturing of ISO PLUS and CGP products
- Advanced protective clothing such as mask, hairnet and gloves for high risk processes such as final weighing steps during the manufacturing of CGP products
- Filtering processes to remove large particles after the peptide purification process during the manufacturing of CGP products
- Peptide pooling and aliquotation are performed in sterile workbenches for CGP products
Capping
Peptides are synthesized stepwise by attaching one amino acid after the other. Since no chemical reaction has a yield of 100%, at each step there is a small percentage of the growing peptide chain, for which the coupling reaction of amino acids is not working. The residual unreacted amino functionalities can then react with the next amino acid in the sequence, thus creating a deletion within the peptide sequence. These deletion sequences could easily lead to false positive responses in your assays (e.g. as de-novo epitopes).
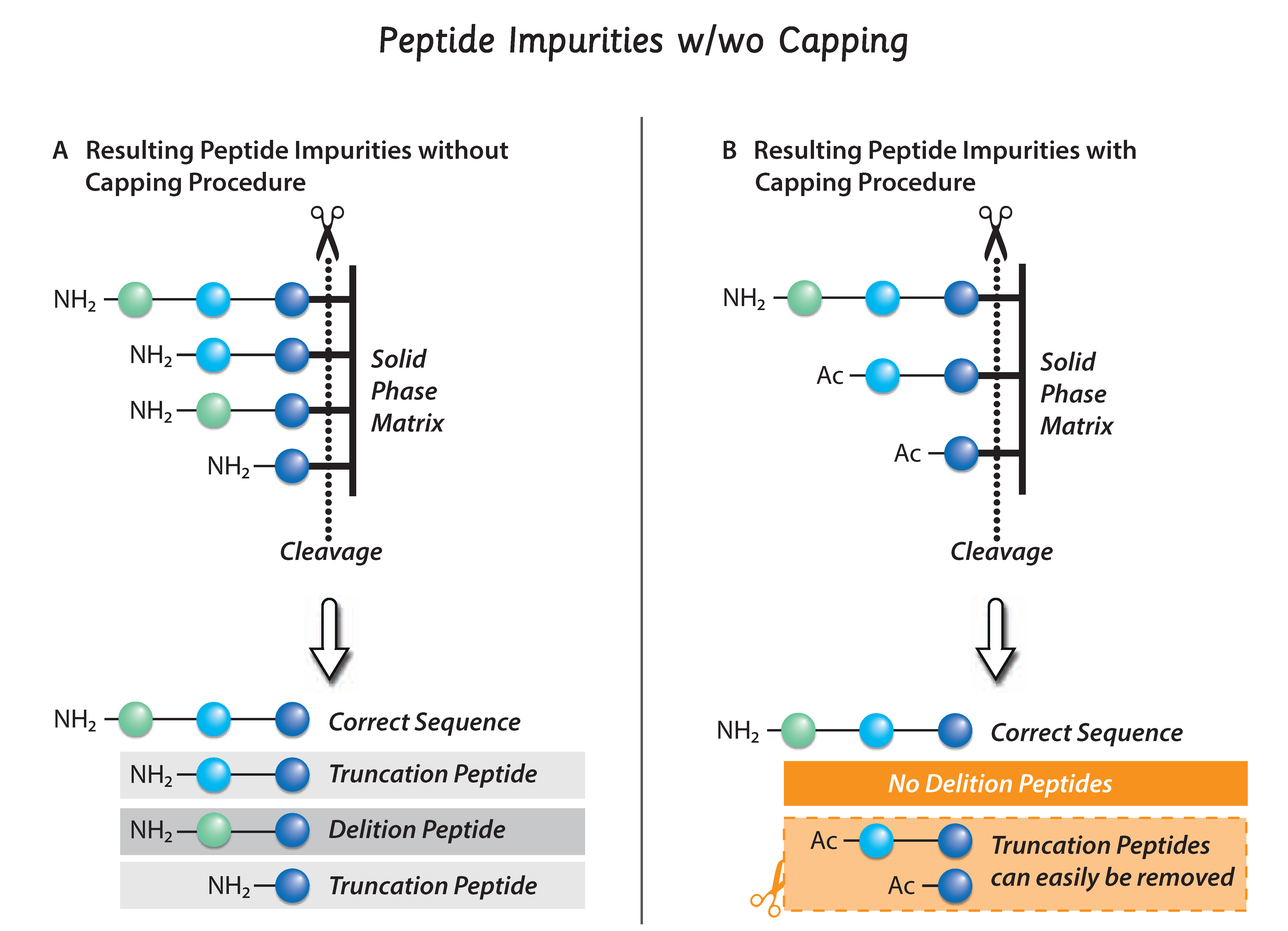
We at JPT developed a proprietary capping method to minimize these deletion sequences. After each step of adding a protected amino acid to the existing peptide sequence, we apply an extra capping step. This eliminates the presence of free amino functionalities by forming capped truncated peptides that are unable to form unwanted deletion peptides.
Although our capping protocol is time consuming and adds to the cost of production, we apply capping for all our peptides regardless of peptide purity!
Here is a video explaining the whole capping process!

Loading...
Looking for further peptide knowledge? Visit
