How to dissolve peptides?
Solubility of Peptides
 Peptides are short chains of amino acids that have a wide range of applications in research, drug development, and therapeutics. After peptide synthesis and purification, peptides are typically delivered as lyophilized powder. Proper peptide solubilization can be challenging and incomplete solubilization affects peptide concentration, which can significantly affect your experiments and results. Here we explore the factors that influence peptide solubility and offer guidelines for improving solubility in practical applications. However, peptide solubility is difficult to predict and you may need to test solubility with a small amount.
Peptides are short chains of amino acids that have a wide range of applications in research, drug development, and therapeutics. After peptide synthesis and purification, peptides are typically delivered as lyophilized powder. Proper peptide solubilization can be challenging and incomplete solubilization affects peptide concentration, which can significantly affect your experiments and results. Here we explore the factors that influence peptide solubility and offer guidelines for improving solubility in practical applications. However, peptide solubility is difficult to predict and you may need to test solubility with a small amount.Factors Influencing Peptide Solubility
Amino Acid Composition: The nature of the amino acids within a peptide sequence plays a critical role in its solubility. Hydrophilic amino acids like lysine, arginine, and glutamic acid increase solubility in aqueous solutions such as buffers, whereas hydrophobic amino acids such as leucine, isoleucine, and tryptophan can decrease solubility. Overall hydrophobic peptides containing many hydrophobic or polar uncharged amino acids can usually be dissolved in organic solvents such as DMSO.
Peptide Length: Longer peptides tend to have lower solubility than shorter ones. The increased number of hydrophobic interactions in longer chains can lead to aggregation and precipitation.
pH and Charge: The total charge of the peptide and the pH of the solution can significantly affect solubility. Peptides with many acidic amino acids can be dissolved in basic buffers, whereas peptides with basic amino acids can be reconstituted in acidic solutions.
Guidelines for Peptide Solubility
Adjust the pH: Determine the charge of your peptide (read more for instructions) and use a solvent with an appropiate pH.
Solubility Testing: Start with solubility tests in small volumes using buffers at different pH levels. This will help determine the optimal conditions for dissolving the peptide without wasting material. We also offer solubility testing among other peptide analysis services if you wish to outsource.
Use of Co-solvents: The addition of organic solvents such as DMSO, ethanol, or acetonitrile in small amounts can improve the solubility of hydrophobic peptides. However, the compatibility of these solvents with subsequent experimental applications should be carefully considered. In general, a maximum of 1% DMSO in the final working solution is acceptable for most assays.
Peptide Modification: Chemical peptide modifications such as the addition of hydrophilic groups or the substitution of hydrophobic amino acids can improve solubility. Common modifications include pegylation or the incorporation of D-amino acids to disrupt aggregation.
Temperature Control: Some peptides are more soluable at higher temperatures. Gradual heating of the solution while monitoring the solubility can be effective, but care must be taken to avoid degradation of the peptide.
Sonication: Sonication helps stirring the solution and improves dissolution.
Centrifugation: Always centrifuge your peptide solution before use to precipitate any undissolved peptide residues.
Dissolving Peptides and Troubleshooting
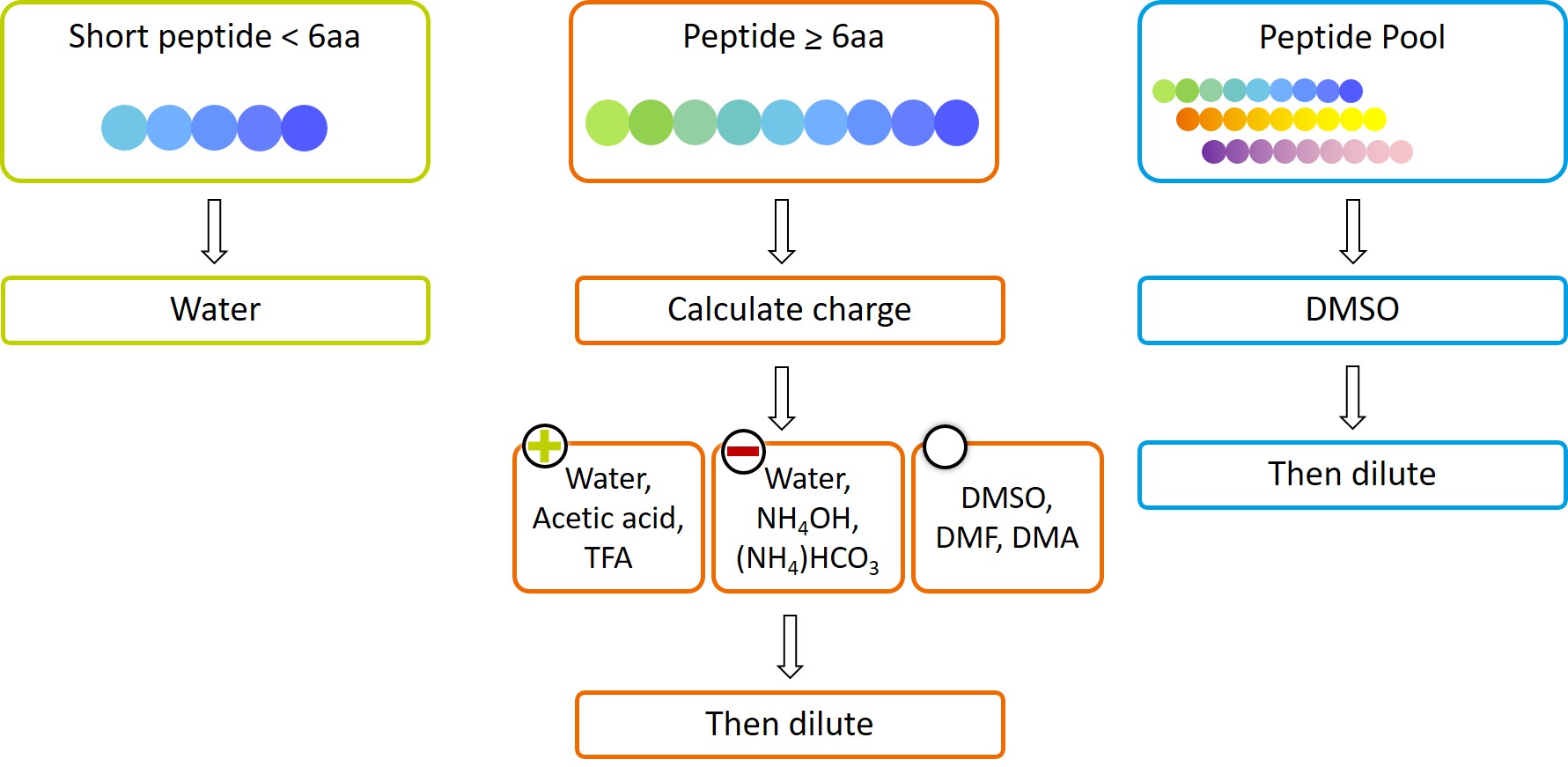
We recommend that you use the required solvent first and then dilute to the desired concentration with water, your buffer or medium.
Always be sure to test solubility first with a tiny amount of peptide and follow these steps:
Short Peptides
If your peptide is very short (< 6 aa), try water as solvent.
Standard Peptides
If your peptide is longer you need to check the amino acid sequence and calculate the total charge of your peptide:
Positively charged peptides: First try water or buffer as a solvent. If this does not work, lower the pH by adding 10% acetic acid solution. You may add a small amount of TFA (< 50 μl) if needed.
- Assign a value of -1 to each acidic residue (D, E, and COOH at the C-terminus).
- Assign a value of +1 to each basic residue (K, R, H and NH2 at the N-terminus).
Positively charged peptides: First try water or buffer as a solvent. If this does not work, lower the pH by adding 10% acetic acid solution. You may add a small amount of TFA (< 50 μl) if needed.
Negatively charged peptides: First try water or buffer as a solvent. If this does not work, increase the pH by adding aqueous ammonia or 10% ammonium bicarbonate solution.
Uncharged & hydrophobic peptides (with > 25% hydrophobic aa): Use a small amount of an organic solvent. We prefer DMSO, but DMF, DMA, isopropanol, or methanol can also be used. In the second step, add water or an aqueous solution stepwise to the desired concentration. Vortex or sonicate after each step.
Be careful not to exceed 1% (v/v) DMSO in your final solution if you are using your peptides in cellular assays!
Note that DMSO should not be used with peptides containing methionine or cysteine as it may oxidize the side chain. Use DMF instead.
Dissolving PepMixTM Peptide Pools
When dissolving PepMix peptide pools, we recommend to prepare a stock solution by dissolving the peptides in dimethyl sulfoxide (DMSO) at room temperature. Typically, using 50 µl of DMSO should be sufficient to dissolve the contents provided of a vial containing 25 µg per peptide. If necessary, aliquots should be prepared immediately, and any remaining stock solutions stored at -20°C or below. The stock solution can be diluted for use in your assay.
Additional sonication may be helpful! Always centrifuge your peptide solution before use to precipitate any undissolved peptide residues.
How to Dissolve Peptides:
Can we help you?
 We offer a solubility testing service based on Turbidimetric Solubility Assay (TSA). Take a look at our peptide solubility testing and other peptide analysis services.
We offer a solubility testing service based on Turbidimetric Solubility Assay (TSA). Take a look at our peptide solubility testing and other peptide analysis services.
If you have any further questions, please contact our experienced support teams!
