Cell Therapy Peptides & Pools
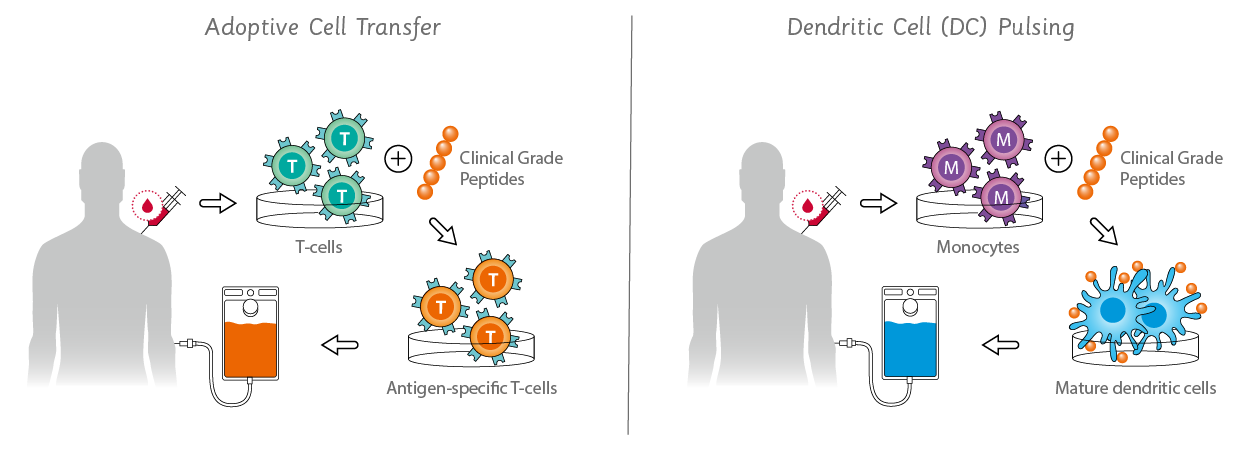
With the recent success of immunotherapy and cell therapies (cytotherapy) the need for clinical grade peptides & peptide pools has grown rapidly. To meet the requirements for peptides in cancer immunotherapy by adoptive cell transfer or dendritic cell pulsing, JPT has established an enhanced production protocol for tailored cell therapy peptides and peptide pools. We produce all of our clinical peptides and pools such as neo-epitope pools, antigen-spanning peptide pools and peptide libraries according to our stringent Clinical Grade or ISO PLUS policies that even exceed the most current DIN EN ISO 9001:2015 regulations.
Peptides in Cell Therapy & Immunotherapy
- High quality synthetic peptides are essential for all immunotherapy development phases
- Individual peptides, peptide pools or peptide conjugates are needed
- Examples include adoptive cell therapy, dendritic cell pulsing and CAR-T cell therapy
Peptides are Better Antigens
- Epitopes or overlapping peptides and pools may mimic protein antigen function
- Neo-epitopes enable personalized therapies
- Peptides can be synthesized in high purities at low bioburden or sterile
- No antigen expression needed, no bacterial contamination, ADCF regulations applicable
- Sequence diversity and post-translational modifications can be addressed
Why Work with JPT?
- The quality of our peptides & customer service are unparalleled
- We have unique peptide formats for discovery, therapy, and immune monitoring
- We offer knowledgeable personal consultation to help you woth peptide specifications
- We have a long track record of successful studies and projects
Clinical Peptides & PepMix™ Pools
PepMix™ Peptide Pools