Peptide Dimers
Peptide dimers or multimers have several advantages over their monomeric counterparts. For example, it is known that dimerization of GPCR ligands can result in a significant increase in affinity. Another example are MAPs (multiple antigenic peptides), where formation of multimers leads to increased immune response (see Linker / Spacer / PEGylations for details).
The chemistry used for peptide dimerization often takes advantage of chemoselective reactions between unprotected peptides. Examples are the formation of the following bonds: Cys-maleimide thioethers, disulfides or triazoles (click chemistry). See the table below for their respective structures. In addition to the depicted chemoselective dimerization methods below, it is also common to dimerize protected peptide fragments (e.g. for the synthesis of dimers connected by amides).
Most Popular Peptide Dimerization Methods
|
Name |
Structure |
|---|---|
|
Cys-maleimide thioether |
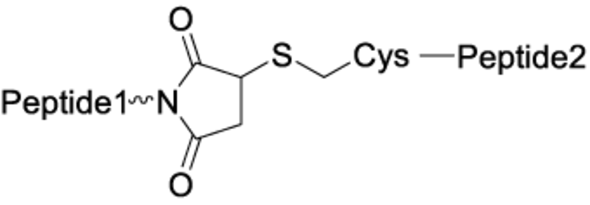 |
| Disulfide |
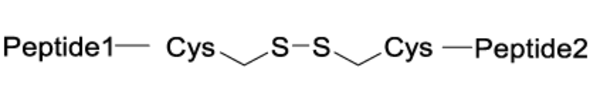 |
| Click chemistry |
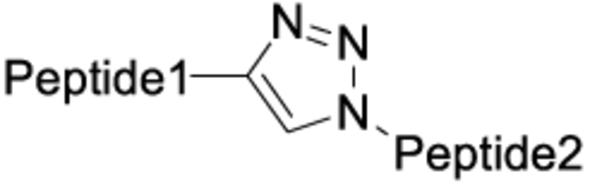 |
| Amide | 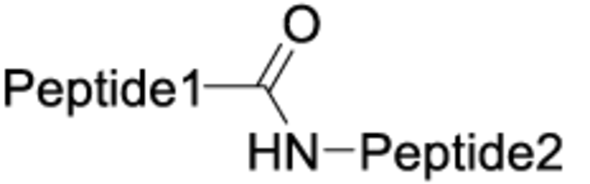 |
