Click Chemistry
"Click chemistry" usually refers to the reaction of an azide with an alkyne yielding 1,5-disubstituted 1,2,3-triazoles. Without additives, this reaction (Huisgen 1,3-dipolar cycloaddition) is relatively slow. However, in 2002 a copper(I)-catalyzed version was reported independently by Meldal and Sharpless. As under copper-catalysis the reaction proceeds quickly and selectively, Sharpless coined the term "click reaction". Another common name for the copper-catalyzed variant is CuAAC (Cu catalyzed alkyne azide cycloaddition).
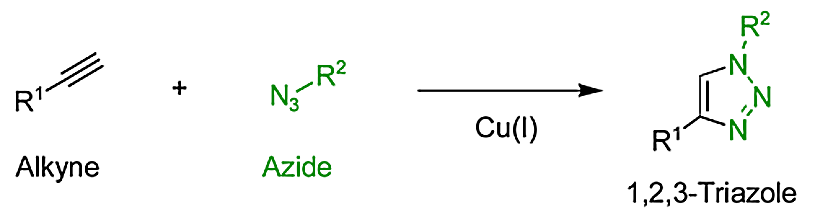
Click chemistry is frequently used in peptide assembly because of several advantages:
- The reaction is fast and quantitative
- The reaction is irreversible
- The reaction is compatible with all functional groups present in natural peptides
- The reaction is compatible with water
- The reaction is pH insensitive (in the range of pH 4-11).
- The triazole moiety formed by the click reaction is similar in geometry and polarity to the amide bond
- The formed triazole is stable (e.g. to oxidation, hydrolysis, enzymatic degradation)
- Azides and alkynes are easy to introduce into peptides
The most important applications of click reactions in peptide chemistry are non-native chemical ligation (e.g. peptide dimerization), peptide cyclization, bio-conjugation of peptides and the synthesis of peptidomimetics. Finally, the click reaction is also a well-suited method for the conjugation of peptides to dyes, PEGs or radioligands.
Due to the cytotoxicity of copper, a copper-free click chemistry method has been developed by Bertozzi et al. This reaction – also called strain-promoted alkyne-azide cycloaddition (SPAAC) – proceeds without copper at low temperature. An unfavorable strain in the alkyne moiety (usually part of a cyclooctyne core structure) is released by reaction with the azide.
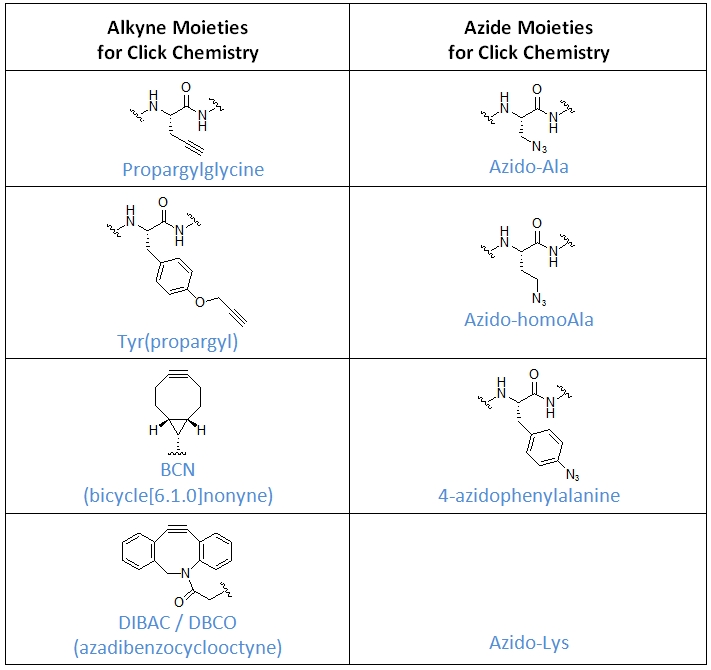
JPT has vast experience performing click chemistry as well as with the synthesis of peptides for click chemistry. The table below shows some examples for the most common building blocks for the introduction of alkynes and azides.